Stainless steel is among the metals used to make cutlery, cookers, and other appliances because of its durability. While stainless steel is known for its properties, it possesses unique characteristics when it comes to thermal conductivity as compared to other metals. This article aims to uncover the standing of stainless steel compared to metals like aluminum, copper, and brass, and explore their values. Different industries like electronics, construction, and manufacturing rely primarily on efficient piece functionalities and heat exchange mechanisms, hence, this information is essential for them. Take a deep breath as an engineer or a designer, for alongside dispelling the doubts regarding metals, this guide explains how the world around us is shaped by metals and the situation regarding thermal conductivity.
What is the Thermal Conductivity of Stainless Steel?
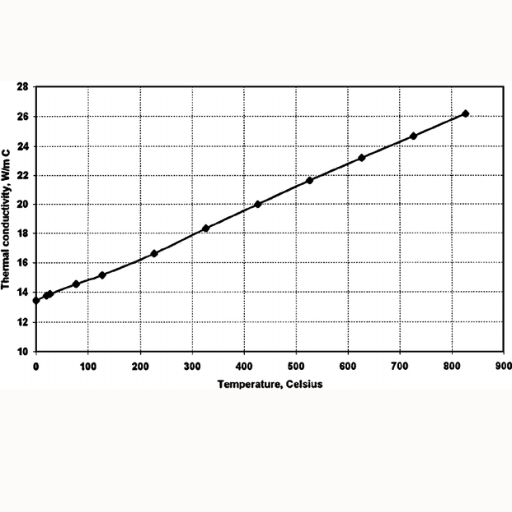
Stainless steel has a lower thermal conductivity than other metals like aluminum and copper. It has a thermal conductivity range of approximately 10 to 30 W/mK, depending on its grade. For instance, grade 304 stainless steel has a thermal conductivity value of 16 W/mK. Although this value is low, stainless steel performs remarkably well in retaining heat or providing thermal insulation. This makes the metal perfect for use in cookware and construction materials.
How does Stainless Steel’s Thermal Properties differ from Carbon Steel’s?
Carbon steel is better than stainless steel at transferring heat and has a higher thermal conductivity. The thermal conductivity of carbon steel ranges between 43 W/mK to 65 W/mK. The stainless steel range of 10 W/mK to 30 W/mK is significantly lower. Stainless steel excels in retaining heat or transferring it in a controlled manner, which is critical for industrial applications. Moreover, stainless steel is less prone to rusting, which is critical for places where the temperature and environment are subject to rapid changes.
Why does Stainless Steel have Low Thermal Conductivity?
| Key Point | Explanation |
|---|---|
| High Alloy Content | Chromium and nickel disrupt heat conduction pathways. |
| Crystal Structure | Different grades (e.g., austenitic, and ferritic) affect conductivity. |
| Low Electron Mobility | Fewer free electrons limit heat conduction. |
| Corrosion Resistance | Alloying elements for corrosion reduce thermal conductivity. |
| Temperature Dependence | Conductivity increases slightly with temperature. |
| Microstructure Variations | Different grades (e.g., austenitic, ferritic) affect conductivity. |
How is the Thermal Conductivity of Stainless Steel Measured?
Most stainless steel thermal conductivity measurements require specialized instruments or methods that analyze heat flow through the material. One such method is the Laser Flash Method which, as the name suggests, utilizes a laser to study thermal properties of metals like stainless steel. The method yields a material’s thermal diffusivity which is then used in conjunction with density and specific heat capacity to derive thermal conductivity through the formula:
k = α × ρ × Cp
Where:
- k – thermal conductivity (W/m·K)
- α – thermal diffusivity (mm²/s)
- ρ – density (kg/m³)
- Cp – specific heat capacity (J/kg·K)
Compared to materials such as copper or aluminum, stainless steel thermal conductivity values are relatively low. For example, the thermal conductivity of austenitic stainless steels (like Grade 304 and 316) is between 14–16 W/m·K at room temperature while ferritic grades might show slightly higher values.
Example Data:
- Grade 304 Stainless Steel:
- Thermal conductivity at ~20°C (68°F): 16.2 W/m·K
- Specific heat capacity (Cp): ~500 J/kg·K
- Density (ρ): ~8,000 kg/m³
- Grade 316 Stainless Steel:
- Thermal conductivity at ~20°C (68°F): 14.6 W/m·K
- Comparison with Copper:
- The thermal conductivity of copper at ~400 W/m·K shows why stainless steel is not widely adopted for applications requiring high heat transfer efficiency.
This information is collected in a laboratory environment with strict control over the temperature and surroundings as they can impact the measurements. The outcomes aid engineers and scientists in determining where the materials can be used, especially where controlled heat transfer and corrosion-resistant features are essential.
How does Stainless Steel Compare to Other Metals Like Copper and Aluminum?
Stainless steel, copper, and aluminum are suitable for different applications due to their distinctive properties. Stainless steel is the least thermally conductive compared to copper and aluminum. For example, copper is one of the best thermal conductors, making it a popular choice in electrical wiring and heat exchangers. Aluminum is less conductive than copper, but its lower weight makes it more desirable for use in cooling systems, as well as in aerospace components. Stainless steel is ideal in settings that require resistance to corrosion, strength, and durability because it is not efficient for tasks that require optimal heat conduction.
What are the Thermal Conductivities of Metals Like Copper and Aluminum?
| Metal | Thermal Conductivity (W/m·K) | Key Notes |
|---|---|---|
| Copper | 385 – 401 | Superior heat conductor. |
| Aluminum | 205 – 237 | Lightweight, cost-effective. |
Why is Stainless Steel a Poor Thermal Conductor Compared to Other Metals?
| Key Point | Explanation |
|---|---|
| High Alloy Content | Chromium and nickel disrupt heat pathways. |
| Crystal Structure | Austenitic structure reduces heat transfer efficiency. |
| Low Electron Mobility | Fewer free electrons limit heat conduction. |
| Corrosion Resistance | Alloying elements for corrosion reduce conductivity. |
| Temperature Dependence | Conductivity increases slightly with temperature. |
What Applications Use Stainless Steel Despite Its Lower Conductivity?
While stainless steel is not as thermally conductive as metals such as copper and aluminum, its strength, durability, and corrosion resistance allow it to be used in a wide variety of applications. Here are five important areas where stainless steel is indispensable.
- Cookware and other kitchen appliances
Stainless steel is a favorite in kitchen appliances due to its ability to resist rust and corrosion. For this reason, sinks, pots, and pans are made of stainless steel, as well as kitchen utensils which need to be robust over long periods and especially in damp conditions.
- Medical devices and surgical implants
Stainless steel’s biocompatible nature and corrosion resistance allow for use in medical blades, surgical tools, and orthopedic implants. Its ability to undergo harsh sterilization procedures without being damaged makes it indispensable in healthcare.
- Construction and Architecture
Is commonly applied for cladding, roofing, and various structural parts of buildings due to its high strength and durability, aesthetic qualities, and resistance to harsh weather conditions which are ideal for decorative and functional appliques.
- Aerospace and automotive industry
Stainless steel’s durability and heat retention make it suitable for parts like fasteners, exhaust systems, and various structural components. It may not be as heat-conductive, but its strength and extreme weather tolerance make it indispensable for these industries.
- Equipment for Food Processing and Chemicals
Because stainless steel is hygienic and resists corrosion from chemicals, acids, and food particles, it is widely employed for processing tanks, piping, and reactors in the chemical and food industries. Its versatility most certainly has its limits in the field of thermal engineering, but it is invariably an asset in countless other areas.
What are the Unique Thermal Properties of Different Grades of Stainless Steel?

The various thermal properties of stainless steel based on its grade, which makes certain types more appropriate for specific uses:
- Austenitic Stainless Steels (e.g., 304, 316): These grades possess appreciable resistivity toward heat, and they can retain strength at higher temperatures, although their thermal conductivity is relatively low. They are frequently employed in regions needing heat resistance, like industrial ovens and heat exchangers.
- Ferritic Stainless Steels (e.g., 430): These grade types have relatively better thermal conductivity than austenitic types, but a lower resistivity to heat. They are widely used in the automotive industry for components like exhaust systems which operate at moderate temperatures.
- Martensitic Stainless Steels (e.g., 410): They present a middle range of heat resistivity and are usually selected for their high strength and wear resistance, for instance, turbine blades.
- Duplex Stainless Steels: Offering a moderate level of thermal conductivity and good strength, these steels are suitable for tough environments such as chemical processing as they combine the features of both austenitic and ferritic steels.
The balance of each grade’s thermal conductivity, resistance, and strength provides great flexibility to engineers and designers to meet different industrial requirements.
How do Austenitic Stainless Steels Differ in Thermal Conductivity?
| Grade | Thermal Conductivity (W/m·K) | Temperature (°C) | Key Notes |
|---|---|---|---|
| 304 | 16.2 | 20 | Commonly used, low conductivity. |
| 316 | 16.2 | 20 | Similar to 304, better corrosion resistance. |
| 316L | 16.2 | 20 | Lower conductivity, high-temperature resistance. |
| 310 | 12.3 | 20 | Lower conductivity, high-temperature resistant. |
| 321 | 15.7 | 20 | Stabilized grade, moderate conductivity. |
| 347 | 15.7 | 20 | Similar to 321, stabilized with niobium. |
| 314 | 17.6 | 20 | Higher conductivity, good for heat applications. |
Comparing 304 Stainless Steel with 316 in Terms of Thermal Conductivity
| Parameter | 304 Stainless Steel | 316 Stainless Steel | Key Notes |
|---|---|---|---|
| Thermal Conductivity | 16.2 W/m·K | 16.2 W/m·K | Both have similar thermal conductivity. |
| Temperature Range | Stable at 20°C to 500°C | Stable at 20°C to 500°C | Conductivity is consistent across the range. |
| Corrosion Resistance | Moderate | High | 316 resists chlorides better. |
| Applications | Food, architecture, medical | Marine, chemical, medical | 316 suited for harsh environments. |
| Cost | Lower | Higher | 316 is more expensive. |
How Does Annealing Affect the Thermal Conductivity of Stainless Steels?
With regards to stainless steels, annealing changes their microstructure, which impacts thermal conductivity. The heating and cooling involved in the annealing process tend to relieve internal stresses, as well as make the structure of the material more uniform. Based on my experience, thermal conductivity can improve slightly, but that would depend on the grade of stainless steel and the annealing steps undertaken. Usually, the improvement is insignificant to the material’s thermal conductivity and alloy composition has a more important influence.
What are the Benefits of Stainless Steel Despite Low Thermal Conductivity?

Even stainless steel’s relatively low thermal conductivity comes with numerous advantages. Its corrosion resistance is crucial in environments that are exposed to moisture, chemicals, and high humidity. Moreover, stainless steel is extremely hard and durable, exhibiting remarkable strength about its weight and ensuring long-lasting performance. In addition to being easy to maintain, stainless steel is also easy to clean. Its hygienic properties make it suitable for food processing as well as medical and industrial applications. Lastly, stainless steel is aesthetic and sustainable because it can be reused for construction or consumer products, thus making it versatile.
How Does Corrosion Resistance Compensate for Low Thermal Conductivity?
Low thermal conductivity is compensated for by corrosion resistance which guarantees reliability and dependability in demanding situations, lower maintenance costs, and enhanced material function over time, making the limitations in thermal efficiency obsolete.
Why is Stainless Steel Preferred in Food Processing?
| Key Parameter | Details |
|---|---|
| Corrosion Resistance | Resists rust, acids, and caustic substances. |
| Hygiene | Easy to clean and sterilize effectively. |
| Durability | Withstands wear, impact, and temperature changes. |
| Non-reactive Surface | Does not alter food taste, color, or quality. |
| Temperature Resistance | Performs well in extreme hot or cold conditions. |
| Bacteria Resistance | Smooth surface prevents bacterial growth. |
| Regulatory Compliance | Meets food safety standards globally. |
| Versatility | Suitable for various food processing equipment. |
What Role Does Architecturally Exposed Structural Steel Play in Modern Design?
Exposed Structural Steel has major significance in contemporary design since it integrates practicality with beauty. I admire how it enables structural components to serve as design elements because they are used as such, making bold innovative statements while preserving architectural strength and stability.
Does Thermal Expansion Affect the Thermal Conductivity of Stainless Steel?

Of course, thermal expansion may alter the conductivity of heat in stainless steel, but the difference is usually negligible. When temperature increases, stainless steel expands, and the distance between its atoms changes (spacings). This change can somewhat affect the material’s heat conduction ability. Although, the impact is not great enough to change thermal conductivity for most use cases. Such reliability makes stainless steel a dependable construction material for places with high and low-temperature extremes.
How Does Thermal Expansion Influence Heat Transfer?
Thermal expansion is a critical factor in how some materials manage heat, especially if they are subjected to regular temperature shifts. For instance, as stainless steel expands, the heat increases its atomic structure which becomes more outspread, thus reducing its density. Although, this can slightly impede heat transfer efficiency, for most practical applications, the impact is usually negligible.
To illustrate, the linear expansion coefficient of stainless steel usually has an alloy-dependent value of 16 to 17 x 10^-6 °C n per degree Celsius (°C). Therefore, with a 100-degree Celsius temperature rise, the 10-meter stainless steel rod could expand by roughly 1.6 to 1.7 millimeters. These figures, while very relevant to the design of a precise engineering system, are not as impactful as the actual engineered system in terms of its heat conduction capabilities.
Thermal expansion still has its considerations in modern research, especially in extremely cold or hot temperature conditions. Components stress or small deformation aggrandizers within components can impede heat transfer internally in expertly designed systems such as pipes or heat exchangers. In these cases, space for thermal expansion is included in the design to ensure proper thermal effectiveness during variable conditions.
How is Electrical Conductivity Related to Thermal Conductivity in Metals?
Metals share both electrical and thermal conductivity due to the movement of free electrons. If I’m not mistaken, metals do conduct electricity and heat well due to the mobility of free electrons within the material. Free electrons relocate not only charge but also thermal energy. The association of these two properties is defined by the Wiedemann-Franz law which says the ratio of thermal to electrical conductivity is proportional to temperature, as long as the metal structure is consistent.
References
- Local thermal conductivity mapping of selective laser melted 316L stainless steel – University of Texas repository.
- Thermal contact resistances in a thermal conductivity test system – Oregon State University library.
- Methods for Improving Thermal Uniformity of Stainless Steel Pedestal Heater – California State University repository.
Frequently Asked Questions (FAQ)
Q: What is thermal conductivity and how does it relate to the ability to conduct heat in metals?
A: Thermal conductivity is a measure of how well a material can conduct heat. It is expressed in watts per kelvin per meter (W/m·K). Metals like copper or aluminum have a high thermal conductivity, meaning they are excellent at conducting heat, while stainless steel has a lower thermal conductivity.
Q: Why does stainless steel have a lower thermal conductivity compared to metals like copper or aluminum?
A: Stainless steel is an alloy composed of iron, carbon, and other elements, which affects its thermal properties. Unlike pure metals like copper or aluminum, the alloy structure of stainless steel results in a lower thermal conductivity, making it a less efficient conductor of heat.
Q: What is the typical thermal conductivity of stainless steel?
A: The thermal conductivity of stainless steel is generally around 15 watts per kelvin per meter, depending on the specific type of stainless steel. This is lower compared to high thermal conductivity metals like copper, which has a conductivity of around 400 watts per kelvin per meter.
Q: How do different stainless steel grades affect thermal conductivity?
A: Different stainless steel grades have varying alloy compositions, which can affect their thermal conductivity. For example, ferritic stainless steels tend to have slightly higher thermal conductivity than austenitic types. However, all stainless steels have lower thermal conductivity compared to metals like copper or aluminum.
Q: In what applications might the low thermal conductivity of stainless steel be advantageous?
A: The low thermal conductivity of stainless steel can be beneficial in applications where heat retention is desired, such as in cookware or heat exchangers. Its ability to maintain structural integrity at high temperatures also makes it suitable for different applications in the automotive and aerospace industries.
Q: How does the type of stainless steel affect its ability to conduct thermal energy?
A: The type of stainless steel, including its alloy composition and structure, can affect its ability to conduct thermal energy. For example, low carbon stainless steels may have slightly different thermal properties compared to high carbon varieties, but all will generally have lower thermal conductivities compared to metals like copper.
Q: Are there any stainless steel grades with higher thermal conductivity?
A: While all stainless steel grades have relatively low thermal conductivity compared to metals like copper, some grades like ferritic stainless steels might offer slightly higher thermal conductivity compared to austenitic grades. However, none will match the highest thermal conductivity found in pure metals.
Q: Can the thermal conductivity of stainless steel be improved?
A: Improving the thermal conductivity of stainless steel typically involves altering its alloy composition or developing new types of steel. However, significant improvements are challenging due to the fundamental properties of the materials involved.
Q: How is thermal energy transported in stainless steel?
A: Thermal energy is transported in stainless steel primarily through lattice vibrations and electron movement. The presence of alloying elements in stainless steel disrupts these processes, leading to its lower thermal conductivity compared to purer metals.
Q: How does the thermal conductivity of stainless steel compare to other steel types?
A: Stainless steel generally has a lower thermal conductivity compared to carbon steel and other steel types due to its alloy composition. However, its unique properties like corrosion resistance and strength at high temperatures make it valuable for specific applications despite its lower ability to conduct heat.