The melting point of 316 stainless steel is a key characteristic determining its capacity to function in various industries. This post will delve into the melting point of 316 stainless steel, its factors, significance, and comparisons with other stainless steels, for example. It will also analyze the material’s performance at elevated temperatures and the reasons for preferring it in harsher conditions. In the end, you will have a well-informed perspective on the melting properties of 316 stainless steel alongside its versatility and durability in varying sectors.
What is the 316 stainless steel melting point?
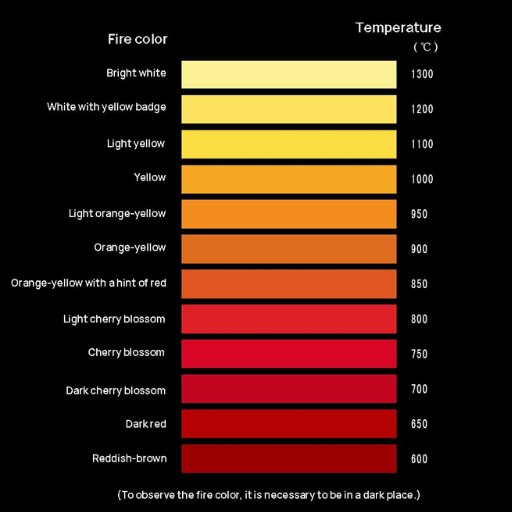
The melting temperature of 316 stainless steel is between ~2500°F and 2550°F (1370°C and 1400 °C). This temperature is high due to its chemical composition: Iron, Chromium, Nickel, and Molybdenum. These elements turn 316 stainless steel into a heat-resisting alloy, making it more suitable for demanding applications.
Understanding the Melting Temperature
According to my research, 316 stainless steel has a melting point between 1,370 °C and 1,400 °C. This estimate is due to the iron, chromium, nickel, and molybdenum it contains. These elements provide heat resistance and protect the material from damage at higher temperatures, enabling it to be used in different fields such as chemical processing, construction, and manufacturing.
The Role of Alloy Composition in Melting Point
The various parts within an alloy significantly impact the melting point and can independently change the composition and properties of the material. For example, in 316 stainless steel, chromium provides oxidation resistance as well as structural stability at higher temperatures. In addition, molybdenum allows for better endurance against pitting and crevice corrosion in chloride surroundings, while nickel improves durability and resistance.
The melting point for 316 stainless steel is 2500 to 2550 degrees Fahrenheit or 1370 to 1400 degrees Celsius. This alloy achieves these relatively high melting points due to its balanced composition of iron as the primary metal alongside 10-14% nickel, 16-18% chromium, and 2-3% molybdenum. Each alloy element is strictly controlled to minimize the structural capability and performance in demanding tasks. Therefore, the exact composition of the alloy is ideal for corrosive and incredibly high-temperature situations encountered in chemical processing and marine engineering, as well as when used in heat exchangers.
Comparing 316 and 304 Stainless Steel Melting Points
The melting points of 316 steel and 304 steel alloys are relatively close, around 1400 to 1450 degrees Celsius or 2550 to 2650 degrees Fahrenheit. However, compared to 304 steel, 316 stainless steel is more suitable for more extreme environments because the molybdenum has increased the steel’s heat resistance and durability.
How Does the Composition of 316 Stainless Steel Affect Its Properties?
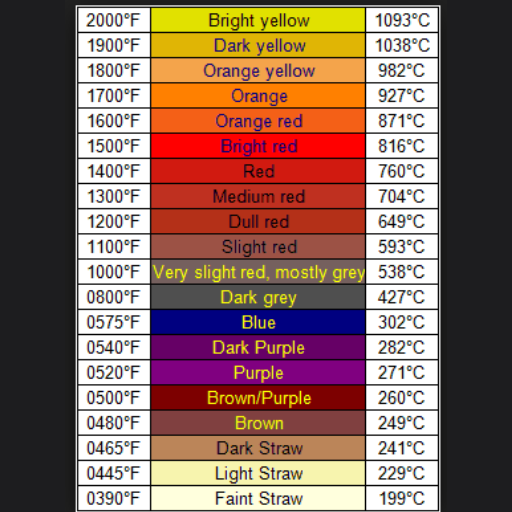
The compounds that makeup 316 stainless steel are much more effective when working independently. These compounds are overly chromium and nickel, which provide corrosion resistance, and molybdenum, which helps withstand pitting and chloride crevice corrosion. Higher chloride concentrations make stainless steel preferred in the marine, chemical processing, and medical industries. This steel is further strengthened due to the unique combination of elements, expressing superlative durability, strength, and temperature resistance.
The Influence of Chromium and Nickel
Your best bet at beating corrosion with steel is chromium since it works with oxygen and gives off a thin protective barrier. This oxidized covering repels water, preventing the steel from rusting. When penetrated by extreme temperatures, nickel allows expansion and contraction of the stem without it wearing off, which improves the steel’s ductility while also improving its strength. These elements allow stainless steel to be deployed in extreme environments with oceanic or chemical industries.
The Impact of Carbon and Other Elements
A plethora of components play an essential role in the durability of 316 stainless steel in the sea. One of those components is carbon, which can either help maintain strength and hardness or work against it by negatively impacting resistance to corrosion. For 316 stainless steel, retaining ≤ 0.08% carbons is standard to resist corrosion and damage while being balanced out by strength.
Molybdenum, manganese, silicon, and phosphorus are some of the other alloys that make up the different features of an alloy. Molybdenum (2 to 3 percent) aids in resisting pitting and crevice corrosion for 316 stainless steel, making it useful in marine applications. Manganese (up to 2%) improves hot workability, and silicon’s impact (less than or equal to 0.75%) assists oxidation resistance at high temperatures. Phosphorus and sulfur typically appear in minimal quantities (less than or equal to 0.045 percent and less than or equal to 0.03 percent, respectively), as some increased values can result in brittleness or a reduced ability to wield it. These separated particles, big in minimum quantity, supply significant units of demanding industrial applications, ensuring the alloys are robust, flexible, and dependable.
Why is 316 Stainless Steel Preferred in High-Temperature Applications?

316 stainless steel is commonly used in high-temperature environments because of its astonishing oxidizing and corrosive qualities. Using molybdenum improves the alloy’s ability to resist chloride environments and prevents pitting, while chromium enhances thermal stability. This allows the alloy to retain mechanical strength and structural integrity at higher temperatures, making it ideal in chemical processing, power generation, and heat exchangers as industry equipment. Its diversity and high durability in harsh environments ensure optimal performance for an extended period.
The Importance of Corrosion Resistance
Corrosion resistance is vital in different industries as it affects the durability and effectiveness of components in harsh conditions. Materials like stainless steel alloys that have high corrosion resistance practically do not corrode due to moisture, chemicals, and high salinity, which averts failures and expensive upkeep. For example, 316 stainless steel alloys with 2-3% molybdenum content are preferred in chloride regions due to their resistance to pitting corrosion. Also, keeping the chrome content at a minimum of 16-18% ensures that a passive oxide layer, which provides thermal stability and protection against oxidation, is formed. With a proper selection of materials paying attention to those technical parameters, the efficiency and performance in seawater systems, chemical plants, and high-temperature industrial processes are guaranteed.
Benefits of Austenitic Stainless Steel in Extreme Conditions
The unique blend of mechanical and chemical properties of Austenitic stainless steel includes corrosion resistance and outstanding performance under extreme environments. Even in harsh conditions like acidic solutions and salt water, the high chromium (16-21%) and nickel (6-25%) content ensures no oxidation and corrosion. Furthermore, additional molybdenum (2-3%) for specific grades like 316 and 316L enhances the resistance to pitting and crevice corrosion, essential for marine and chemical processing applications.
Austenitic stainless steel also offers impressive toughness while remaining durable at cryogenic temperatures and scrolling and thermal fatigue resistance at high temperatures, making it suitable for the extreme 1500F (815C). These features enable austenitic stainless steel to be the most favorable option when constructing heat exchangers, pressure vessels, and industrial plant components. Moreover, its magnetic non-properties and excellent weldability assure flexibility in various engineering fields.
With unmatched material strength, superior corrosion resistance, and unmatched flexibility, austenitic stainless steel is the most reliable option to ensure long service life and low maintenance for demanding applications.
What are the Applications of 316 Stainless Steel in Industry?

316 stainless steel is used in almost every industry because of its high resistance to corrosion, strength, and durability. It is often seen in chemical and petrochemical equipment, marine applications, pharmaceutical and food processing facilities, and heat exchangers. Due to its high strength, it is also used in medical and device components, surgical instruments, and automotive parts. The alloy is also used in construction architecture for structural supports, railings, and cladding where corrosive weather or agents are present.
Usage in Chemical Processing and Aerospace
Stainless steel or high-performance alloys are commonly used in the chemical processing and aerospace industries due to their impressive mechanical properties and ability to withstand extreme conditions.
Chemical Processing
Stainless steel 316 and Hastelloy blends are widely used in chemical processing. They are known to have high resistance to corrosion from acids, alkalis, and chlorides, allowing them to endure a great deal of punishment. For example, stainless steel 316 can tolerate temperatures up to 1,500F (815C) and work with corrosive materials such as sulfuric or hydrochloric acid at controlled concentrations. Moreover, these materials are extensively used in reactors, tanks, piping, and valves, where safety and operational effectiveness depend on reliability.
Aerospace
The aerospace industry consumes materials of a high strength-to-weight ratio that can endure extremely high temperatures and pressures. Widely used alloys include titanium and nickel-based superalloys such as INCONEL® and aluminum-lithium. For example, INCONEL 718 is an alloy with superb strength at temperatures between -423F to 1,300F (-253C to 705C), making it suitable for use in jet engines, turbines, and spacecraft components. In addition, these materials have better fatigue and creep resistance and can withstand constant mechanical and high-speed stress without losing structural integrity.
These alloys and stainless steel have become synonymous with performance, especially in demanding environments. Their use in chemical processing and aerospace technologies improves advancements in both industries.
Applications in Welded Components
Superalloy welded components are crucial in sectors with high mechanical properties and corrosion resistance. Superalloys are often used to fabricate heat exchangers, pressure vessels, and high-temperature piping systems. Their use in petrochemical plants, power generation facilities, and aerospace applications is widespread because they tend to have excellent weldability and strength retention at elevated temperatures.
Essential Functions or Measures Relating to a Welded Component
- Heat Input Control: Heat input during welding is an area of concern regarding microstructure. The recommended values for heat input are commonly around 1.0 to 2.5 kJ/mm, which may very well depend upon the material’s thickness.
- Pre-Heating and Post Weld Heat Treatment (PWHT): Preheating for PWHT between 100F -300F (38C – 149C) decreases thermal stress while ductility and residual stress rest over 1,800F – 2,200F(982C -1,204C)
- Shielding Gas Composition: Shielding with high-purity argon provides precise arc stability while preventing oxidation on the welded surfaces. This is critical for ensuring proper shielding.
- Weld Filler Material Selection: Base materials for welded structures tend to be expected to match or exceed the resistance properties of the base filler materials. ERNiCrMo-3 for superalloys aids in common operations.
Engineering today is marked by great reliability and durability, with components expertly shielded from the terrors of a corrosive, high-stress atmosphere.
How Does Heat Treatment Affect the Melting Point?

The melting point of any material is an intrinsic value and is defined by the atomic structure of the material. That being said, heat treatment cannot directly alter the value. Nevertheless, the heat treatment process can change the microstructure of the materials, in addition to the mechanical properties and the phase distribution. These changes may affect how the material behaves around the melting temperature. Tempering or hardening will change the strength and ductility of the material, giving high performance at elevated temperatures while the melting point remains unaffected. An example of beat treatment is an anneal that will homogenize the material.
Changes in Microstructure Due to Heat Treatment
The microstructure of any given material is changed via heat treatment and directly affects the physical and mechanical properties of the material. A form of heat treatment called annealing provides a more uniform microstructure and the ability to reduce internal stresses, alleviating workability issues and increasing ductility. In contrast, hardening has the opposite effect, increasing the percentage of martensite in steel. While the hardness and strength of the steel increases, ductility tends to decrease. After hardening, tempering is conducted to make the microstructure less brittle and able to maintain stability at less than the same level. Unlike the more specific requirements, these processes provide optimized performance with the material’s melting point unchanged in high-temperature or load-bearing situations.
Effect on Mechanical Properties and Durability
I know that when dealing with mechanical characteristics and the effect of time, processes like hardening and tempering impact steel’s proficiency. Hardening increases strength and wear resistance while making the material less ductile. Grylls outlines the case in which tempering increases toughness and extends the lifespan while countering deceptively high levels of brittleness. For guidelines where operating conditions involve a lot of psychological pain in stress and lesser decompression, excellent durability near retractable is highly recommended, making these changes crucial. Generally, these types refine the net result so that operational requisites on steel are very far from gentle.
References
- TBK Metal: Understanding the Melting Point of 304 and 316 Stainless Steel
- Industrial Metal Supply: Comparing 304 & 316 Stainless Steel
- Metal Supermarkets: What Is the Melting Point of Stainless Steel?
Frequently Asked Questions (FAQ)
Q: What is the melting point of 316 stainless steel?
A: The melting point of 316 stainless steel is typically between 1371 and 1399 degrees Celsius (2500 and 2550 degrees Fahrenheit). This range is due to the composition of steel alloys, which include elements like chromium and nickel.
Q: How does the melting point of stainless steel compare between grade 304 and grade 316?
A: The melting point of 304 stainless steel is generally similar to that of grade 316, but 316 is often preferred for its better corrosion resistance, particularly in marine applications. Both grades have a melting range of around 1400 degrees Celsius (2550 degrees Fahrenheit), but due to its composition, 316 may offer enhanced performance at high temperatures.
Q: Why is 316 stainless steel commonly used in marine applications?
A: Grade 316 stainless steel is commonly used in marine applications due to its superior corrosion resistance compared to other types of stainless steel, such as grade 304. This is primarily because 316 contains molybdenum, which enhances its ability to withstand harsh marine environments.
Q: What are the main differences between 304 and 316 stainless steel?
A: The main differences between 304 and 316 stainless steel are their composition and corrosion resistance. 316 has a higher nickel content and includes molybdenum, which provides increased resistance to chlorides and acids, making it more suitable for chemical processing and marine environments than 304.
Q: How does the composition of the steel affect the melting point?
A: Stainless steel’s composition, including the amounts of chromium, nickel, and other elements, affects its melting point. Different grades of stainless steel have varying compositions, which result in slightly different melting ranges. For example, the presence of nickel in 316 allows it to maintain structural integrity at high temperatures.
Q: What are the applications of grade 316 stainless steel?
A: Grade 316 stainless steel is used in various applications that require high corrosion resistance and strength, including chemical processing, food processing, and marine applications. Its ability to withstand high temperatures and harsh environments makes it ideal for these purposes.
Q: Is 316 stainless steel considered a type of 18-8 stainless steel?
A: Yes, 316 stainless steel is referred to as a type of 18-8 stainless steel because it contains approximately 18% chromium and 8% nickel. However, it also includes molybdenum, distinguishing it from the more commonly known type 304 stainless steel.
Q: How do oxidation and corrosion resistance relate to the melting point of stainless steel?
A: Oxidation and corrosion resistance do not directly affect stainless steel’s melting point but are important properties for its performance at high temperatures. The composition of stainless steel, including its high chromium and nickel content, helps prevent oxidation and maintains integrity under heat, allowing it to retain its melting range and structural properties.
Q: Can 316 stainless steel be used at high temperatures?
A: Yes, 316 stainless steel can be used at high temperatures due to its stable composition and melting range. These properties allow it to maintain its properties and resist oxidation, making it suitable for applications involving heat and corrosive environments.







