Thermal conductivity is one of metals and alloys’ most important physical properties. Industries that deal with electronics, automotive design, construction, or energy systems require a deep understanding of thermal conductivity. Knowing which material to choose is especially important when heat management is critical, as it immensely impacts efficiency, performance, and durability. Whether you are an engineer, a designer, or a merely interested enthusiast, this article will guide you through the essentials, the actual uses, and the features that determine material selection based on heat conduction.
What is the Thermal Conductivity of Stainless Steel?
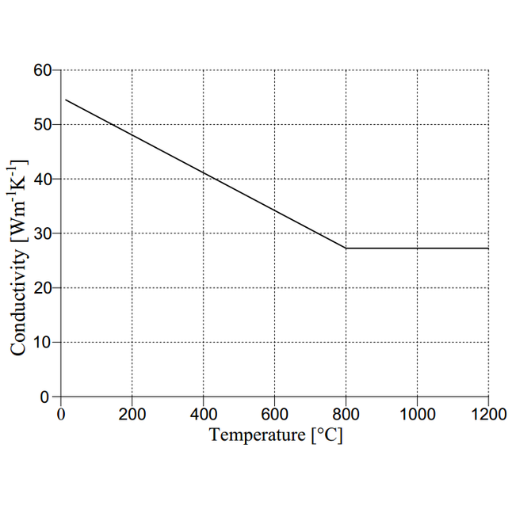
Compared to metals like copper and aluminum, stainless steel has a lower thermal conductivity. Its thermal conductivity is about 15 to 25 W/m·K (watts per meter-kelvin) at room temperature, depending on the grade and alloy composition. These values are pretty low for stainless steel, so it is often used where good heat conduction is not wanted, such as in thermal insulation.
Factors Affecting Thermal Conductivity in Metals
The main factors affecting the thermal conductivity of metals are atomic structure, mobilities of electrons, temperature, and material purity. Metals such as copper and silver possess high thermal conductivity primarily due to their free electron density and resistance to flow. Copper, for example, has a thermal conductivity of about 398 W/m·K at room temperature, while silver exceeds this value, reaching approximately 428 W/m·K. This makes the two metals ideal for electrical wiring and heatsinks, which require efficient heat transfer.
On the other hand, stainless steel has metals with low thermal conductivity due to its structural composition and additional alloying elements such as chromium and nickel. These elements hinder the flow of electrons and thermal conductivity.
A Comparison of Thermal Conductivity in Common Metals
| Metal | Thermal Conductivity (W/m·K) | Applications |
|---|---|---|
| Silver | 428 | High-performance electronics, thermal interfaces |
| Copper | 398 | Wiring, heat exchangers, and thermal management systems |
| Aluminum | 237 | Lightweight heatsinks, radiators, and aerospace |
| Stainless Steel | 15-25 | Cookware, insulation, and controlled heat transfer systems |
| Titanium | 21 | Aerospace components, medical implants |
From the above comparison, one can conclude that a metal’s thermal properties significantly dictate its hindered value in thermal applications. Although silver and copper lead in thermal conductivity, stainless steel is favored when thermal resistance and durability are preferred.
Importance of Material Selection
Thanks to technological advancements, comprehensive material data is available, and selecting metals for thermal applications is very precise. High thermal conductivity metals are valuable in applications where heat dissipation is needed; low thermal conductivity metals, on the other hand, are used in cryogenic or controlled heating environments where heat transfer needs to be minimized. This shows the overwhelming importance of thermal conductivity in system design, considering efficiency and sustainability.
Factors Affecting the Thermal Conductivity of Stainless Steel
| Factor | Impact on Thermal Conductivity |
|---|---|
| Alloy Composition | Chromium reduces, and nickel has a minimal effect. |
| Microstructure | Austenitic has lower, ferritic has higher conductivity. |
| Temperature | Conductivity increases with temperature rise. |
| Processing Methods | Cold rolling decreases, and annealing increases conductivity. |
| Surface Conditions | Smooth surfaces enhance, oxidation reduces conductivity. |
| Environmental Factors | Corrosion or humidity can reduce conductivity. |
How Does Carbon Steel Compare in Thermal Conductivity?

Compared to stainless steel, carbon steel has a lower thermal conductivity. Therefore, it is not as effective in transferring heat and does not work well for applications where heat needs to be dissipated efficiently. However, lower thermal conductivity works to carbon steel’s advantage when retaining heat or requiring controlled heat transfer. Higher carbon content as part of the composition sets carbon steel apart from stainless steel in exhibiting these thermal properties.
Key Differences Between Carbon Steel and Stainless Steel
| Aspect | Carbon Steel | Stainless Steel |
|---|---|---|
| Main Alloy | Iron and carbon | Iron, chromium (≥10.5%), and nickel |
| Corrosion Resistance | Poor | Excellent |
| Strength | Higher in high-carbon variants | Strong, varies by grade |
| Ductility | Lower in high-carbon variants | Higher, especially in austenitic grades |
| Cost | Generally cheaper | More expensive |
| Magnetic Properties | Magnetic | Some grades are non-magnetic |
| Appearance | Dull, prone to rust | Shiny, rust-resistant |
| Applications | Structural, tools, and machinery | Medical, food, marine, decorative |
The Role of Carbon Content in Thermal Properties
| Carbon Content | Thermal Property Impact |
|---|---|
| Low Carbon (<0.3%) | Higher ductility, lower thermal conductivity. |
| Medium Carbon (0.3-0.6%) | Balanced strength, moderate conductivity. |
| High Carbon (>0.6%) | Increased hardness, reduced heat dissipation. |
| Heat Treatment | Enhances strength, alters thermal behavior. |
| Thermal Conductivity | Decreases with higher carbon content. |
Applications Benefiting from Carbon Steel’s Thermal Conductivity
- Manufacturing of Cookware
Carbon steel is used in industrial works, fryers, and baking sheets because of its controllable thermal conductivity. It is also helpful for evenly distributing heat, which is needed when preparing food. In addition, carbon steel’s heat retention is perfect for meat searing and temporary cooking.
- Heat Exchangers
Carbon steel is used in industrial processes as a construction material for heat exchangers due to its steady thermal conductivity and robust construction. The material also improves the heat transfer rate between fluids, preventing structural damage during pressurized situations.
- Boiler Systems
High-performance carbon steels are used for boiler systems in power plants and industrial workplaces because they efficiently handle precise heat exchange. The components also withstand structural wear, making them long-lasting in high-temperature environments.
- Radiators and Heating Elements
District and local heating systems with carbon steel radiators ensure cost-effective and sufficient heating. These components maintain the effectiveness of heat distribution within residential and commercial spaces.
- Automotive Parts
Carbon steel’s thermal retention and conductivity make it a good construction material for exhaust systems and other engine parts, enabling high-performance vehicles to operate and endure extreme temperatures.
What are the Thermal Properties of Different Metals?
Different metals possess unique thermal properties that impact their uses:
- Copper
Copper ores are excellent conductors of heat, and they have one of the highest thermal conductivity values among metals. Thus, they are widely used in constructing electrical wiring and sinks.
- Aluminum
Due to its lightweight, aluminum has high thermal conductivity, making it suitable for use in heatsinks and aerospace components.
- Steel
Steel has good thermal conductivity and retains heat well. It is used in structural and automotive industries where thermal stability is vital due to moderate heating.
- Gold and Silver
These two metals have extremely high thermal conductivity. They are mainly used in specialised applications, like electronics, where gold and silver are only used because of their high price.
- Titanium
Titanium is lower than other metals in thermal conductivity, but has exceptional heat resistance, making it ideal for use in aerospace and medical equipment.
The combination of these factors determines each metal’s role in the industry and technology, including its cost, conductivity, and resistance to heat.
Comparing Thermal Conductivities Across Metals
According to the most recent information, silver and copper possess the highest thermal conductivity, while titanium and stainless steel possess very low thermal conductivity. This makes them ideal for applications that need strength and resistance to heat.
Metals with High Thermal Conductivity and Their Uses
Copper, aluminum, and other metals are crucial in many practical applications. Regarding personal preference, I like copper the most because it is used in electrical wires and heat exchangers due to its great thermal conductivity and strength. Aluminum’s low weight and ability to transfer heat effectively also make it an excellent candidate for cooling systems and parts for aircraft. These characteristics allow the two metals to perform critical functions in industries that need reliable heat control and structural integrity.
How is Thermal Conductivity Measured in Metals and Alloys?

The thermal properties of metals and alloys are determined by examining the passage of heat through the material. The most common approach utilizes either steady-state or transient methods.
- Steady-State: This approach drives heat to flow through a material and measures the amount of heat transferred while keeping the temperature of the boundaries constant. Thermal conductivity is determined from the material’s heat flow, geometry, and relative temperature difference.
- Transient: This method measures the temperature change of an object or a material after applying an external stimulus, such as a laser beam, and determines how the change in the heat input affects its temperature. Instruments like the laser flash apparatus measure thermal diffusivity, which is then multiplied by specific heat and density to obtain thermal conductivity.
In measuring any physical characteristic of a material, homogeneity, uniform temperature, and identical properties within the temperature range across the measurements must be maintained.
Advanced Methods for Measuring Thermal Conductivity
Improved techniques for measuring thermal conductivity are being developed due to the accuracy and reliability provided by new technologies. Here are some of the changes from recent years:
- 3-Omega Method: This method is standard for measuring the thermal conductivity of thin films and nanostructured materials. Through this method, the thermal conductivity of small-scale materials can be derived from the measurement of phase delay and amplitude of a thermal wave caused by alternating current in a thin metallic filament. Measurements from this method are highly accurate for silicon and graphene, with thermal conductivity values often in the range of hundreds W/m·K, e.g., 150 W/m·K for silicon at room temperature.
- Time-Domain Thermoreflectance (TDTR): TDTR can measure heat diffusion in materials by monitoring the time it takes using ultrafast lasers. TDTR excels at layered and nanostructured materials; recent works highlight its ability to measure the thermal conductivity of thin films as low as 0.05 W/m·K for aerogel materials.
- Microelectromechanical Systems (MEMS) Sensors: MEMS-based devices have been created, focusing on incorporating microscale sensors to evaluate the shape and size of small composite materials’ thermal properties. Current studies on these MEMS systems demonstrate heightened detection sensitivity, which has been used to measure the thermal conductivity of polymer composites and even biological samples.
- Laser Flash Analysis of Complex Materials: Laser techniques have been expanded to include measuring at extreme industrial and extraterrestrial pressures and temperatures. Newer data indicate that materials such as aluminum nitride can exceed 200 W/m·K thermal conductivities at elevated temperatures, making this method important for evaluating high-performance materials.
Incorporating these advanced techniques takes the understanding of thermal conductivity of different materials to a whole new level. These methods will undoubtedly continue to revolutionize research and aid scientists and engineers in designing and optimizing materials for energy, electronics, and thermal management systems.
Understanding Watts per Kelvin per Meter as a Measure
Measuring thermal conductivity in watts per kelvin per meter (W/m·K) indicates the material’s engineering potential to conduct heat. This measurement demonstrates the heat energy transmitted through a material within a stipulated distance and along a thermal gradient. This measurement is necessary in many highly technical applications requiring absolute precision in thermal management.
Here is a table of materials versus their thermal conductivity values (W/m·K)
- Copper – 385 W/m·K (High conductivity found in electronic components and other places where heat exchange is done.)
- Silver – 430 W/m·K (Best thermal conductor known; used in exceptional cases like the high-efficiency solar panels.)
- Aluminum – 205 W/m·K (Used widely because it is lightweight and performs well.)
- Diamond – 2200 W/m·K (Best known thermal conductor; utilized in high technology and systems needing rapid heat removal.)
- Glass – 1.05 W/m·K (Has low conductivity and so is principally used in glasses for windowing and as part of insulation systems.)
The figures explain the various capabilities of materials in different branches of industries and technologies. Depending on thermal engineering requirements, these materials allow more profound insight into material discrimination.
Challenges in Accurate Thermal Conductivity Measurements
Measuring thermal conductivity accurately can be difficult because of the measurement sensitivity and different materials’ behavior. The contributing factors to these challenges are the following:
- Material Homogeneity
A material’s thermal conductivity can change depending on its composition and structure. Homogeneous materials yield more uniform results, while heterogeneous ones, like composites, show localized differences. For instance, fiber-reinforced composites exhibit anisotropic thermal conductivity, which complicates their measurement.
- Measurement Methodology
Not all techniques are flawless, as is the case with steady-state techniques such as the guarded hot plate method and transient techniques like laser flash analysis or the hot-wire method. Steady-state techniques demand strict control of environmental parameters, while transient techniques depend on unrealistic assumptions for boundary conditions.
- Temperature Dependency
Thermal conductivity is primarily temperature-dependent, particularly with metals and polymers. Take copper, for example, a metal known to have high thermal conductivity. It exhibits a dip during cryogenic temperatures. On the other hand, polymers tend to exhibit non-linear increases with rising temperatures. For aerospace and electronics, determining values for wide temperature ranges is a critical undertaking.
- Preparing the specimen
Correct specimen preparation is critical to obtaining precise measurements. Properties such as surface roughness, thickness, and geometry can impact the results. Small imperfections can alter heat flow and introduce deviation. For instance, a thermal barrier coating may be applied to a thinner specimen used for measurement, which is not straightforward.
- Environmental factors
Data might change due to the specimen’s surroundings, such as humidity, oxidative conditions, and pressure. For instance, materials with phenomena like aerogels with thermal conductivities of approximately 0.013 W/m·K show extreme sensitivity to moisture, which can impact the results if not controlled appropriately.
Examples of Data and Innovations in Measurement Techniques
Recent advances in measurement tools aim to solve multifaceted problems. Some of these are based on nanotechnology, such as TDTR, which allows researchers to determine the thermal conductivity of remarkably thin films and nanomaterials, which is critical for modern-day electronics. For example, some works demonstrate that graphene has exceptional in-plane thermal conductivity over 2000 W/m·K, though validating such sophisticated data under diverse conditions is far from simple.
Furthermore, Innovative approaches in machine learning techniques are providing the opportunity to model data, which is predicted to be highly useful in estimating the thermal properties of materials purely based on their constituents, thus minimizing extensive physical tests. This is tremendously useful in designing new composite materials or synthesizing rare-earth insulating compounds.
Along with others, these remain fundamentally important for the advancement of material science. Accurately measuring thermal conductivity, in particular, propels advances in energy storage, thermal management of electronics, and thermal protection systems for spacecraft.
What Are the Unique Thermal Properties of Metals?

All metals have special properties that allow them to possess multiple applications. Their effectiveness as thermal and electric conductivity ensures that heat is absorbed and transferred rapidly. This is mainly because metals have an abundant supply of electrons that move within their structure, making the transfer of thermal energy simple. Moreover, solid substances such as metals tend to have high melting points, making them able to withstand extreme temperatures. These properties are why metals are used in the production of heat exchangers, appliances used in kitchens, and used in managing the heat of electric devices.
Exploring Thermal Diffusivity and Thermal Expansion
Thermal Diffusivity
Thermal diffusivity is a material’s ability to conduct thermal energy proportionately to how well it can store it. This determines the speed of heat flow in a material. High thermal diffusivity materials adjust to temperature changes faster and are ideal in places with large amounts of heat that need quick dissipation. Thermal diffusivity includes the following:
- It is defined mathematically as the thermal conductivity divided by the density and specific heat capacity multiplied together.
- Materials with High Thermal Diffusivity:
- Copper- This material has a high thermal conductivity of approximately
401 W/m·K, making it an excellent thermal diffusivity material. - Aluminum- A low specific heat and moderate density evenly balances energy transfer, making it less diffusive.
- Materials with Low Thermal Diffusivity:
- Ceramics—Some have high thermal conductivity, while most ceramics have low density and specific heat capacities, which results in low diffusivity.
- Polymers are most well-known for being used in the insulation industry due to their low thermal diffusivity.
- Application Example: Heat exchangers in Albop industrial setups benefit from high-diffusivity metals like copper and aluminum.
Thermal Expansion
Drying and leaving a material unattended for too long causes it to overheat, leading to thermal stress. This also increases the size of something as it gets hot. The vibration increase of its tiny parts is caused by or made in a single material. Syncing and keeping track of the heat is crucial to thermal engineering, as material is nearly always subjected to temperature changes. These are some of the most critical features for thermal expansion as they are a crisscross:
- Linear Expansion Coefficient:
- The change rate of the linear graph or dimension is determined by the numbers associated with temperature changes.
- The linear expansion coefficient of steel is about 11-13 Change Per Unit Metre of Heat.
- Volumetric Expansion:
- The term is synonymous with liquids, whereby the entire volume will likely increase. For example, when water is heated, particularly when boiling, it expands across a massive range, but sometimes its practicality in engines and pipelines limits where it loses its practicality.
- High Expansion Materials:
- Materials that fall under this category are known for shrinking with heat, such as polymers like polyethylene.
- Expansion of materials tends to be above a quarter rate of 23 Change Per Unit Metre of Heat.
- Low Expansion Materials:
- Used for practicality in measuring precision engineering, quartz glass and invar alloys are known to have minimal expansion.
- Applications:
- Parts such as gateways and railways are inserted to control how the image stretches, causing the stretch in parts to be controlled.
- When designing electronics, they purposely create a thermal stress zone due to heat and cold stretching and compressing cycles.
Analyzing thermal diffusivity alongside expansion allows engineers and scientists to devise innovative solutions that guarantee durability and efficiency across multiple industries.
Impact of Density and Specific Heat on Thermal Conductivity
| Parameter | Impact on Thermal Conductivity |
|---|---|
| Density | Higher density increases heat transfer efficiency. |
| Specific Heat | Higher specific heat stores more thermal energy. |
| Thermal Effusivity | Combines density, specific heat, and conductivity. |
| Material Composition | Affects density and specific heat properties. |
| Temperature Influence | Alters specific heat and conductivity relationships. |
The Relationship Between Electrical Conductivity and Thermal Conductivity
| Aspect | Key Points |
|---|---|
| Wiedemann-Franz Law | Links thermal and electrical conductivity in metals. |
| Proportionality | Thermal conductivity is proportional to electrical conductivity. |
| Lorenz Number | Constant ratio for metals, ~2.45×10⁻⁸ WΩK². |
| Mechanism | Free electrons transport both heat and charge. |
| Exceptions | Non-metals like diamond break this correlation. |
References
- Thermal Conductivity of Stainless Steel – densem.edu
A detailed explanation of stainless steel’s thermal conductivity. - Thermal Conductivity – AIP Handbook (MIT)
Covers thermal conductivity in metals and alloys using the Wiedemann-Franz-Lorenz law. - Thermal Conductivity – UMich MSE
Discusses thermal conductivity mechanisms in materials, including metals.
Frequently Asked Questions (FAQ)
Q: What is thermal conductivity, and why is it essential in metals?
A: Thermal conductivity measures a material’s ability to conduct heat. It is essential in metals because it affects how well they can transfer thermal energy, which is crucial in applications like heat sinks and thermal conductors.
Q: How is the thermal conductivity of different metals measured?
A: The thermal conductivity of different metals is typically measured in watts per kelvin per meter (W/m·K). This unit indicates how much thermal energy is transferred through a material per unit of time, per unit of temperature difference, and unit of material thickness.
Q: Which metal has the highest thermal conductivity?
A: Silver has the highest thermal conductivity of all metals, with a thermal conductivity of approximately 429 W/m·K. However, copper and aluminum are more commonly used due to their good thermal conductivity and cost-effectiveness.
Q: How does carbon steel compare in terms of thermal conductivity?
A: Carbon steel’s thermal conductivity is around 45 W/m·K, which is lower than that of metals like copper and aluminum. This lower thermal conductivity makes it less efficient for heat transfer applications than metals with higher thermal conductivity.
Q: What are the benefits of stainless steel’s lower thermal conductivity?
A: Stainless steel’s lower thermal conductivity can benefit applications requiring thermal insulation or controlled heat transfer, such as in cooking utensils or thermal expansion management.
Q: Why do copper and aluminum have good thermal conductivity?
A: Copper and aluminum have good thermal conductivity due to their atomic structure, which allows for efficient thermal energy transfer. This makes them ideal for applications requiring high thermal conductivity, such as heat sinks and electrical wiring.
Q: What are the unique thermal properties of alloys compared to pure metals?
A: Alloys can be engineered to have tailored thermal properties that offer advantages over pure metals, such as enhanced strength or corrosion resistance, while maintaining an acceptable thermal conductivity level for specific applications.
Q: How does the ability to conduct heat differ among different metals?
A: Metals’ ability to conduct heat varies significantly. Some, like copper and aluminum, have high thermal conductivity, while others, like stainless steel, have lower thermal conductivity, affecting their suitability for various applications.
Q: What is the relationship between electrical and thermal conductivity in metals?
A: There is often a correlation between electrical and thermal conductivity in metals due to the movement of electrons. Like copper, metals with high electrical conductivity typically also have high thermal conductivity.







