Jewelry is a unique way of expressing one’s style, but sometimes it can make surprising marks, like a ring of discoloration on your skin. If you’re wondering if stainless steel jewelry can have this effect, you’re not the first to ask. With time, many jewelry lovers have become concerned about the different materials used from a skin health perspective. This article looks into whether stainless steel jewelry can leave a nasty green stain in the form of a ring. We will examine why the discoloration occurs, how stainless steel fares are compared to other metals, and how you can shield your skin and your cherished jewelry pieces. Continue reading to get the answers you need and put your mind at ease!
Why Does Stainless Steel Jewelry Sometimes Turn Skin Green?

Stainless steel jewelry is mostly hypoallergenic and very unlikely to cause skin discoloration. However, it can infrequently turn skin green under some specific conditions. The condition arises when the jewelry incorporates small amounts of copper and other alloys with the steel. These metals can react with sweat, skin oils, and moisture, oxidizing to produce a residue that leaves greenish marks. Typically, this is found in low-end or low-grade stainless steel jewelry.
To avoid this, use good-quality stainless steel, keep your jewelry dry, and maintain it properly to reduce interactions.
What Causes Skin Discoloration from Jewelry?
Jewelry is known to cause skin discoloration mainly because of the metal content and the wearer’s skin type. This usually involves alloys of metals, particularly copper or nickel, which are commonly found in jewelry pieces. These metals can oxidize with moisture, oils, or the naturally acidic environment of the skin. The oxidation process can lead to tarnish or residue, which mostly discolors the skin, which is green or black.
Informed studies or expert opinions attribute copper alloys as the most common cause of green stains. When copper comes into contact with water or sweat, it also forms copper salts like copper carbonates or copper chloride, which happen to be green and thus, will transfer to the skin. Lastly, other metals used in jewelry, such as nickel, pose not just a discoloration but allergic reactions in certain sensitive people, which is commonly called Nickel Dermatitis. It is reported that close to 18 percent of people are likely to suffer from nickel sensitivity, which is alarming.
The pH level of your skin also matters a lot. The more acidic a person’s skin is (lower pH), the more likely he/she is to accelerate the corrosion of metals, thus increasing the likelihood of discoloration. Other external factors, such as high humidity or exposure to certain chemicals, such as lotions, perfumes, and some cleaning agents, could also worsen the reaction.
To avoid or lessen skin discoloration due to being in contact with jewelry, experts suggest choosing those made of stainless steel, titanium, or platinum for the best hypoallergenic quality, as these won’t react with the skin. Also, coatings such as rhodium plating can prevent the metal beneath from reacting with the skin, thus serving as protective shields. Jewelry that is not worn for long periods of time should be kept in clean conditions. Moisture should also be avoided for prolonged periods. These could help improve the condition of the skin and jewelry.
Is Nickel in Stainless Steel a Concern?
Yes, nickel can be a problem for people with nickel allergies in stainless steel jewelry. At the same time, general stainless steel does have nickel, parts like surgical steel do not because they are meant to be hypoallergenic. In my view, people using jewelry or other items made of these grade materials are safe, but considering how sensitive one is, one must avoid these items and use them only if no irritation occurs.
How Does Oxidation Affect Metal?
The generally accepted principle about metals and oxidation is that metals oxidize when they react with oxygen. If a type of metal rusts, the result is unsightly corrosion, while other metals may tend to form a protective oxide layer.
How to Prevent Stainless Steel from Turning Green

To stop stainless steel from turning green, follow these steps:
- Keep Clean: Regularly wash the surface of stainless steel using gentle soap. This will remove the stubborn grease, dirt, and oils that may contribute to discoloration.
- Completely Dry: Let the stainless steel surface dry completely. This will prevent moisture from lingering, as prolonged dampness will lead to oxidation.
- Avoid Abrasive Chemicals: Do not use strong bleach, cleaners, or abrasive chemicals. These harsh acids may strip away protective layers on stainless steel.
- Use Protective Coatings: Apply stainless steel polish or sealant to guard against humidity and salt. This provides a barrier against the things that may damage stainless steel and tarnish the surface.
- Proper Storage: Store stainless steel items in a cool, dry place so they are not exposed to corrosive elements, moisture, and prolonged water.
These simple maintenance steps will allow stainless steel to retain its polished look and resist tarnishing for many years.
Can a Protective Layer Help?
Indeed, adding a protective layer will reduce discoloration or cause corrosion while greatly improving stainless steel’s protective qualities and long life. A protective layer acts as a shield that protects the metal from external damage while reducing the impact on its surface. Given below are five major advantages of applying a protective layer on stainless steel:
- Prevention of Oxidation: A protective coating reduces stainless steel’s exposure to oxygen and moisture, the two leading causes of rust and green discoloration.
- Reduces the Corrosion of Chemicals: It helps to prevent the exposure of salt, acids, and industrial pollutants, which are highly corrosive.
- Aesthetic Improvements: Enhancing the beauty of stainless steel with polishing agents or sealants is simple because they provide a shiny, polished finish.
- Lowers Maintenance Work: Cleaning becomes less strenuous with a protective coating because dirt, grime, and fingermarks can no longer stick to the surface of the stainless steel.
- Improved Lifespan in Extreme Conditions: A protective layer significantly increases the lifespan of stainless steels kept outdoors or exposed to the marine environment.
Protective products must be regularly applied to keep stainless steel in good condition. Proper care will ensure it remains in good condition regardless of the environmental conditions it is exposed to.
What are the Best Care Practices for Stainless Steel Jewelry?
| Key Point | Description |
|---|---|
| Regular Cleaning | Use mild soap and water, a soft cloth. |
| Avoid Harsh Chemicals | Keep away from bleach, chlorine, and alcohol. |
| Polish Occasionally | Use a polishing cloth for shine. |
| Remove During Activities | Avoid wearing during sports or swimming. |
| Store Properly | Use soft pouches or lined jewelry boxes. |
| Avoid Abrasive Materials | No steel wool or harsh scrubbing pads. |
| Dry Thoroughly | Prevent water spots or tarnish. |
| Clean After Use | Wipe with a microfiber cloth post-wear. |
| Avoid Frequent Water Exposure | Limit prolonged contact with water. |
| Test Cleaning Methods | Try on small areas before full cleaning. |
Are Certain Activities More Likely to Turn Your Skin Green?
Yes, some activities can increase the chances of the wearer’s skin turning green while wearing jewelry, especially if the jewelry is made of copper or has copper alloy parts. The green coloring, which is a mark of corrosion, comes about because of a process known as oxidation, where metals interact with moisture or an acidic substance on the skin.
For instance, perspiring while exercising can have a profound impact. Salts and oils in sweat can worsen the reaction between your skin and the jewelry. Chlorinated and saltwater pools have the same effect because chlorine and salt can also react with certain metals.
Furthermore, applying makeup and lotion can worsen the effect because they contain certain chemicals that increase the reaction rate. Extremely hot climates can increase the surface area for oxidation, increasing the metal’s oxidation rate.
A study conducted in 2023 mentions that 20% of frequent wearers of copper jewelry, such as fashion pieces or inexpensive rings, reported marked skin discoloration, typically with prolonged contact during water or sweat-filled activities. The good side is that this reaction is nonviolent (not dangerous), and no long-term harm is caused, as it can simply be scrubbed away with soap and water.
To counter these reactions, change the metal used for crafting the jewelry, and stainless steel, platinum, and gold which are known to be less reactive metals can also be used. Otherwise, adding a clear protective coating or gold plating layer can create a barrier between the metal and the skin.
Is Stainless Steel Hypoallergenic and Safe for Sensitive Skin?

Stainless steel is hypoallergenic and safe for sensitive skin. Surgical stainless steel contains lower-grade stainless steel’s nickel content, hence it is more skin-friendly. Hence, hypoallergenic grade stainless steel is ideal for people with sensitive skin.
What Makes a Metal Hypoallergenic?
| Key Point | Description |
|---|---|
| Definition | Unlikely to cause allergic reactions. |
| Nickel-Free | Avoids nickel, a common allergen. |
| Common Hypoallergenic Metals | Platinum, titanium, rhodium, sterling silver. |
| Rhodium Plating | Protects against nickel exposure. |
| Pure Metals | Higher purity reduces allergy risk. |
| Avoid Stainless Steel | Contains nickel, not fully hypoallergenic. |
| Gold Purity Matters | 24k gold is hypoallergenic; alloys may not be. |
| Copper and Brass | Hypoallergenic, but may oxidize skin. |
| Durability | Hypoallergenic metals are often durable. |
| Label Verification | Check for “nickel-free” or hypoallergenic. |
How Does Chromium Contribute to Corrosion Resistance?
| Key Point | Description |
|---|---|
| Formation of Passive Layer | Creates a thin, stable chromium oxide layer. |
| Barrier Protection | Prevents oxygen from reaching the underlying metal. |
| Self-Healing Properties | Oxide layer reforms if damaged. |
| Minimum Chromium Content | Requires at least 10.5% for effectiveness. |
| Resistance in Aggressive Environments | Protects against chlorides and acids. |
| Improves Alloy Durability | Enhances strength and corrosion resistance. |
| Prevents Pitting Corrosion | Reduces localized corrosion in harsh conditions. |
| Maintains Surface Appearance | Oxide layer preserves the metal’s luster. |
| Electrochemical Stability | Reduces anodic reactions in corrosive settings. |
| Widely Used in Stainless Steel | Essential for stainless steel’s properties. |
Can Chemical Reactions Be Prevented?
Avoiding chemical reactions, particularly ones that accelerate corrosion, hinges on precise material and technique choice and adequate environmental controls. Here are five reliable methods to prevent chemical reactions.
- Protective Coatings
Covering a surface with paint, powder coatings, or other non-reactive material like epoxy can effectively block access to moisture, oxygen, or other agents that promote corrosion.
- Cathodic Protection
Corrosion of relatively unimportant components can be protected using a sacrificial anode or impressed current.
- Material Selection
Stainless steel, titanium, and some alloys require little maintenance and repair as they are highly corrosion-resistant.
- Environmental Control
Maintenance of low moisture levels, salt, or acidic conditions greatly decreases the rate of corrosive chemical reactions. Controlled atmospheres and dehumidifiers are often employed in storage facilities.
- Scheduled maintenance, along with thorough cleaning
Dirt, salt, and other contaminants can inhibit accelerated chemical reactions, so periodic cleaning is highly advisable.
What Should You Do If Stainless Steel Jewelry Turns Skin Green?
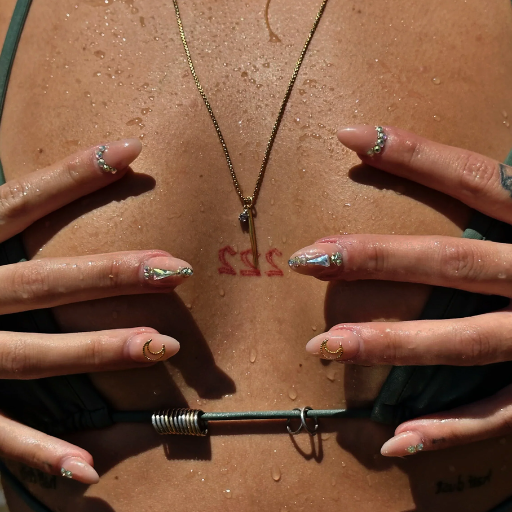
Suppose stainless steel jewelry turns your skin green. In that case, it is likely because of a reaction between specific metal components of the jewelry, like copper alloys, and moisture, sweat, or other factors. Here is what you can do:
- Jewelry Care: Soak the jewelry in lukewarm water while adding cleaning detergent. This will remove any contaminants. Ensure that the jewelry is adequately dried before wearing it.
- Jewelry Protection: Apply clear jewelry-safe nail polish on the metallic sides of the jewelry which will act as a barrier for the skin. It may require constant reapplication.
- Limit Moisture: To reduce a sweat-laden atmosphere, limit exposure to highly active environments like swimming, exercising, and excessive lotion application.
- Higher Quality Stain-Free Metal: Stain-free metals branded as hypoallergenic, known to have higher durability, can be advisable.
Following the procedures outlined above will reduce irritation while wearing stainless steel jewelry and restrict marks on the skin.
How to Remove Green Discoloration?
Jewelry affects skin discoloration, and treating green discoloration can be achieved through gentle scrubbing with soap and warm water and drying with a soft cloth.
Are There Any Home Remedies?
Some aids I’ve come across include home remedies. Lemon juice or vinegar in small amounts can be applied using a cotton ball. Rinse with hot water afterward. Discoloration can also be scrubbed away with a paste like baking soda and water. Do not forget to moisturize afterward to avoid skin dryness.
When is it Time to Seek Professional Help?
If the emotional, physical, or behavioral changes have significantly impacted daily activities, relationships, or coping skills, one should seek professional help.
Comparing Stainless Steel with Other Metals
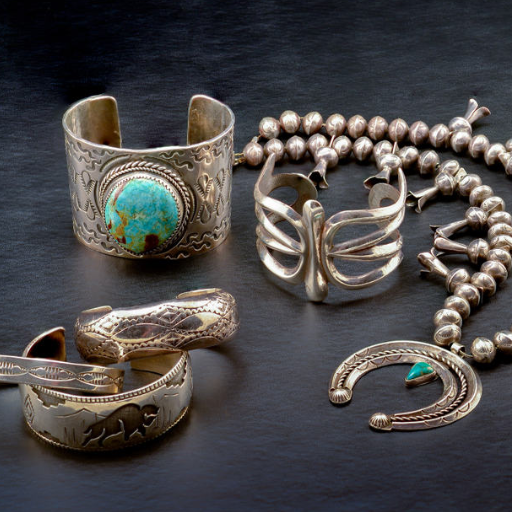
Compared to other metals, corrosion resistance, low maintenance, and durability are the distinctive attributes of stainless steel. Chromium in stainless steel makes it resistant to rust, unlike regular steel. This quality makes it perfect for moist environments. Aluminum is resistant to rust and lightweight, but it decreases durability and is more prone to denting. While copper is valued for its conductivity, it is prone to tarnishing and requires more upkeep, reducing its overall value. Overall, the unique strength, versatility, and longevity make stainless steel preferred for numerous applications.
How Does Stainless Steel Compare to Sterling Silver?
| Key Point | Stainless Steel | Sterling Silver |
|---|---|---|
| Durability | Highly durable, scratch-resistant | Less durable, prone to scratches |
| Corrosion Resistance | Resists corrosion, does not tarnish | Tarnishes over time |
| Maintenance | Low maintenance, easy to clean | Requires regular polishing |
| Appearance | Modern, sleek, high-shine | Warm, lustrous, classic |
| Weight | Heavier | Lightweight |
| Hypoallergenic | Often hypoallergenic (surgical grade) | May cause allergies (depends on alloy) |
| Cost | More affordable | Higher cost, precious metal |
| Design Flexibility | Limited intricate designs | Ideal for detailed, ornate designs |
| Longevity | Long-lasting, everyday use | Can last generations with care |
| Value | Functional, practical | Higher value, heirloom potential |
Is Brass More Likely to Turn Your Skin Green?
Yes, you’re more prone to skin discoloration with brass than with other metals due to some of its composition and the chemical changes it undergoes when dampness and sweat are present—the green discoloration results from copper oxidation, a dominant element of brass. Chemical changes shift in the skin due to contact, and the acids and oils mixed with humidity or wet conditions intensify the problem. The following are five facts and figures that require attention.
- Copper Component
Brass is an alloy mostly made of copper (60-85%) and zinc. It tends to oxidize easily and leaves traces of copper salts, such as copper chloride and copper carbonate, on the skin, manifesting as a green mark.
- Reactions with Sweat and Wetness
Skin oils and sweat contain acids or base elastomers that readily mix with brass, increasing the rate of oxidation, which leads to green staining.
- Surrounding Area Humidity
Brass tends to quickly oxidize with moisture, and high temperatures and humidity tend to worsen the situation. Women can wear these adornments in cold areas, but not during physical exercises with metallic jewelry or in hot, wet conditions, especially when a person has just exercised.
- Protective Coatings
Brass items devoid of protective coatings like lacquer or clear sealant undergo oxidation more readily, thus increasing the chances of skin contact, which causes green stains.
- Skin pH Levels
People with more acidic skin pH are more likely to react with brass. The acidic environment accelerates oxidation, heightening the chances of discoloration.
Considering these factors, actions can be taken to lessen the green-stain effect, such as protecting the brass items with a coating or selecting different metals altogether.
What About Copper and Skin Discoloration?
| Key Point | Description |
|---|---|
| Cause of Discoloration | Reaction of copper with skin and moisture. |
| Common Color | Green discoloration due to copper patina. |
| Harmlessness | Discoloration is not harmful to health. |
| Prevention | Keep jewelry dry and clean regularly. |
| Barrier Methods | Use wax, clear nail polish, or coatings. |
| Cleaning | Wash discoloration with soap and water. |
| Body Chemistry Impact | Skin pH and sweat can influence the reaction. |
| Avoid Chemicals | Remove jewelry before using lotions or cleaning products. |
| Jewelry Material | Pure copper and alloys are more reactive. |
| Maintenance | Regular polishing reduces tarnish effects. |
References
- Carolily Jewelry Blog – This source explains the properties of hypoallergenic stainless steel and its resistance to causing skin discoloration.
- The Cary Company – This article provides an in-depth analysis of stainless steel’s composition and its potential to cause skin discoloration under specific conditions.
- Artizan Joyeria Blog – This blog discusses the factors that lead to skin discoloration from jewelry and highlights stainless steel’s hypoallergenic properties.
Frequently Asked Questions (FAQ)
Q: Can stainless steel jewelry turn your skin green?
A: Typically, stainless steel jewelry does not turn your skin green. This is due to stainless steel’s corrosion resistance and its general non-reactive nature. However, under certain conditions, it might react slightly, but this is rare.
Q: Why might stainless steel jewelry cause skin to turn green?
A: If a piece of jewelry is not pure stainless steel or is mixed with other metals, it might cause a green tint. In some rare cases, reactions with lotions or perfumes might cause it to turn green.
Q: Is stainless steel jewelry hypoallergenic?
A: Yes, hypoallergenic stainless steel is commonly used to make jewelry. This type of steel is less likely to cause skin irritation or allergic reactions, making it a safe option for most people.
Q: How does stainless steel’s corrosion resistance help prevent green skin?
A: Stainless steel’s corrosion resistance helps prevent tarnish and oxidation, which usually causes skin discoloration when wearing jewelry.
Q: Are there specific grades of stainless steel that are more likely to turn green?
A: Higher grades of stainless steel, like surgical steel, are less likely to cause skin discoloration. Lower grades might contain more nickel or copper, increasing the chance of a reaction.
Q: How can I care for my stainless steel jewelry to prevent discoloration?
A: To maintain your stainless steel jewelry, clean it regularly with mild soap and water. This helps prevent any build-up of residues that might react with your skin.
Q: Can wearing stainless steel jewelry cause skin irritation?
A: Generally, wearing stainless steel jewelry does not cause skin irritation due to its hypoallergenic nature. However, individuals with specific metal sensitivities might experience mild irritation.
Q: How do I know if my jewelry is made of stainless steel?
A: Quality stainless steel jewelry is usually marked with a grade, like 316L or 304. Checking for these markings can help ensure your piece is made with genuine stainless steel.
Q: What should I do if my stainless steel jewelry turns my finger green?
A: If your stainless steel jewelry turns your finger green, it might be due to a reaction with another metal in the alloy or external factors like lotions. Try cleaning it and avoiding exposure to harsh chemicals.
Q: Can I wear stainless steel jewelry daily without turning green?
A: Yes, you can wear stainless steel jewelry daily without turning green. Its durability and resistance to tarnish make it an excellent choice for daily wear.